QN-247: A Highly Potent and Differentiated Anti-Cancer Drug Candidate
Originally developed in a collaboration between Qualigen Therapeutics and the University of Louisville, QN-247 is a new chemical entity containing anti-nucleolin oligonucleotide AS1411 covalently bonded to a gold nanoparticle (GNP). Owing to their excellent properties compared to conventional antibodies, notably their smaller physical size and lower immunogenicity and toxicity, oligonucleotides deliver therapeutic drugs by targeting specific cancer-associated hallmarks. Oligonucleotides can also be structurally modified to enable conjugation with other agents, such as nanomaterials like GNPs, thus extending their applications for cancer therapy1. Next, recent breakthroughs in nanoscale manufacturing allow for the potential functionalization of GNPs that could passively distribute through the body, selectively localize in tumors (which are characterized by leaky blood vessels) and be safely excreted through the urinary system2. QN-247 augments the targeting and anti-proliferative properties of oligonucleotides with the distribution capabilities of GNPs to create an potential investigative cancer treatment. The proposed mechanism underlying QN-247 appears below:
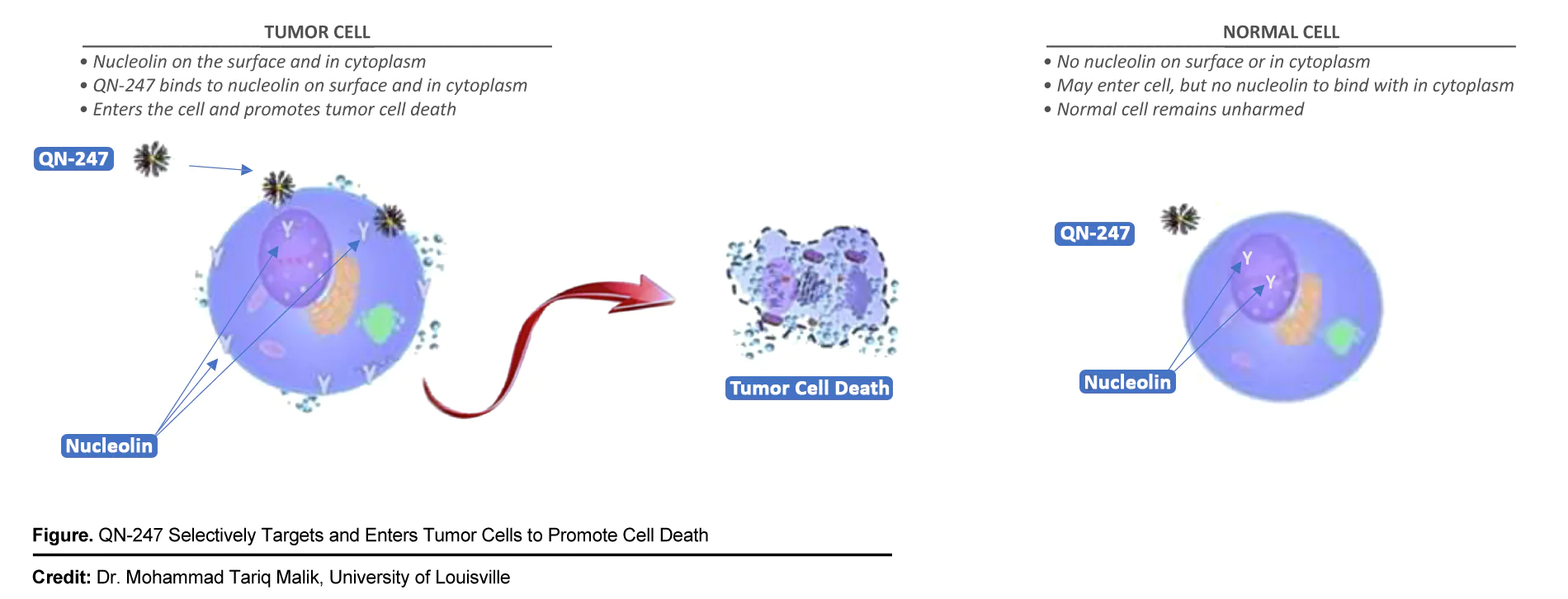
Efficacy Against Triple Negative Breast Cancer (TNBC)
TNBC lacks expression of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2), and accounts for 15% of breast cancers –almost 200,000 cases—worldwide annually3. Compared to other types of breast cancer, TNBC is more aggressive and has a higher risk of recurrence. Likewise, many treatments for breast cancer target hormone receptors and are relatively less effective against TNBC. For these reasons, survival for patients with metastatic TNBC is worse than for other breast cancer subtypes, with a 5-years survival rate of only 12%4. There is an urgent need for better treatment options for TNBC patients.
An in vivo efficacy study with a TNBC MDA-MB-231 xenograft mouse model was performed with 12 daily doses (1 mg/kg) of QN-247. There were statistically significant reductions in mean tumor volumes for all QN-247 formulations compared to baseline (Day -1) and to vehicle control. AS1411 alone did not reduce tumor volume compared to baseline. Moreover, QN-247 formulations with higher oligonucleotide loading appeared to reduce tumor volumes more than lower oligonucleotide loading. No evidence of adverse toxicity was observed. Additional in vivo data is available under CDA.
Efficacy Against Acute Myeloid Leukemia (AML)
Leukemia is a cancer of blood-forming tissues, including bone marrow. AML is the most common form of leukemia and makes up 1% of cancers. There are 20,240 diagnoses per year in the US, out of which around 500 are children5. The 5-year survival rate is 26% for adults and 68% for children6. The current standard of care for AML comprises intensive chemotherapy regimens, biologic agents, and stem cell transplantation. Despite recent advances, therapeutic options are mostly limited to cytotoxic chemotherapy such as cytarabine, which can cause serious toxicity-related side effects. Likewise, recent advancements in targeted therapies such as FLT3 inhibitors target a minority of patients. QN-247 may target a significant portion of AML patients due to the ubiquitous nature of cell-surface nucleolin expressing cancer cells.
Unlike chemotherapies, QN-247 may be able to bind selectively to the surface of cancerous cells. QN-247 significantly reduced the live cell ratio of KG1 cells, an AML cell line, compared to AS1411 or PEGylated gold particles alone. Furthermore, QN-247 may offer a differentiated mechanism that would address the limitations of these therapies. Prior phase 2 clinical data for AS1411 indicated that AS1411 + cytarabine had a complete response rate of 21% compared to 0% for cytarabine alone in relapsed/ refractory AML. The addition of the GNP may increase circulation, improve half-life and delivery to the tumor to enhance efficacy in vivo.
Potential Advantages
- In vivo TNBC xenograft studies show that systemic administration of QN-247 significantly inhibited tumor growth7
- Inhibits growth across a variety of in-vitro cancer cell lines, including AML (KG1), TNBC (MDA-MB-231), and Prostate Cancer (DU-145)
- Greater potency and more robust anti-cancer response in vivo than oligonucleotide AS1411 alone
- Biodistribution studies show tumor-selective accumulation of QN-247 versus normal tissue7
- Selectively binds to cell-surface nucleolin presented on cancer cells
- IP protected by composition of matter with method of use claims
Please contact [email protected] for more information.
Posters & Publications
- Poster – AACR Annual Meeting, April 2023
Nano-immunotherapy: Efficacy of nanoconjugate QN-247 in a Triple Negative Breast Cancer (TNBC) mouse model - Research Article – Royal Society of Chemistry (pubs.rsc.org), October 2019
AS1411 aptamer-modified theranostic liposomes co-encapsulating manganese oxide nano-contrast agent and paclitaxel for MRI and therapy of cancer - Research Article – Nanomaterials (ncbi.nlm.nih.gov), May 2019
Targeted Gold Nanoparticle–Oligonucleotide Contrast Agents in Combination with a New Local Voxel-Wise MRI Analysis Algorithm for In Vitro Imaging of Triple-Negative Breast Cancer - Research Article – Oncotarget (ncbi.nlm.nih.gov), September 2015
AS1411-conjugated gold nanospheres and their potential for breast cancer therapy
References
- Aptamers: A promising chemical antibody for cancer therapy. Oncotarget. 2016 Mar 22; 7(12): 13446–13463. Published online 2016 Feb 3. doi: 10.18632/oncotarget.7178
- Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem., 05 April 2019. https://doi.org/10.3389/fchem.2019.00167
- Anders, C. K., & Carey, L. A. (2022, December 28). ER/PR negative, HER2-negative (triple-negative) breast cancer. Waltham, MA, United States of America. Retrieved from https://www.uptodate.com/
- National Cancer Institute. (2022, December 28). SEER*Explorer. USA. Retrieved from https://seer.cancer.gov/
- Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019 Jul;36:70-87. doi: 10.1016/j.blre.2019.04.005. Epub 2019 Apr 29. PMID: 31101526.
- https://www.cancer.net/cancer-types/leukemia-acute-myeloid-aml/statisticshttps:/www.cancer.net/cancer-types/leukemia-acute-myeloid-aml/statistics
- AS1411-conjugated gold nanospheres and their potential for breast cancer therapy. Oncotarget. 2015 Sep 8;6(26):22270-81. doi: 10.18632/oncotarget.4207. PMID: 26045302; PMCID: PMC4673162.